
Perfusion decellularized hepatic wound matrix primed for cellular integration
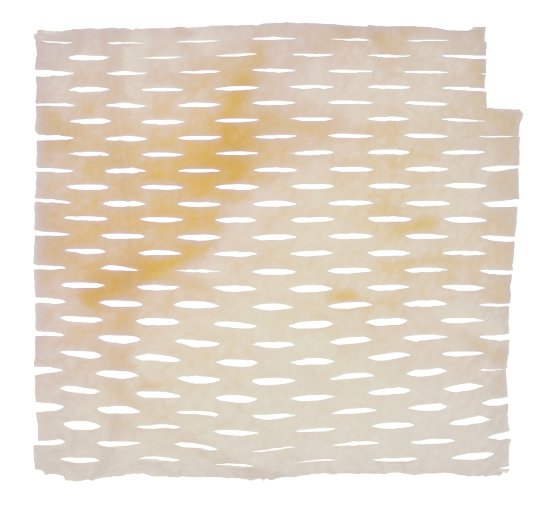
Designed for the management of advanced wounds
- Our proprietary perfusion decellularization process results in an open collagen matrix with natural vascular pathways to assist in cellular infiltration and integration
- Offered in different sizes to meet the individual patient’s clinical needs
Broad range of clinical uses
MiroDerm is a non-crosslinked acellular wound matrix derived from highly vascularized porcine liver. It is intended for the management of wounds, including partial and full-thickness wounds; pressure ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds; drainage wounds; and surgical wounds.
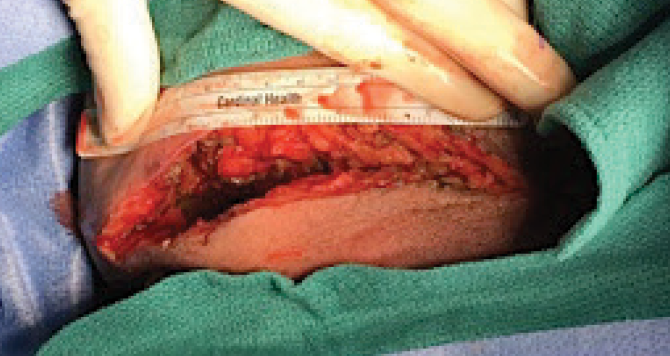
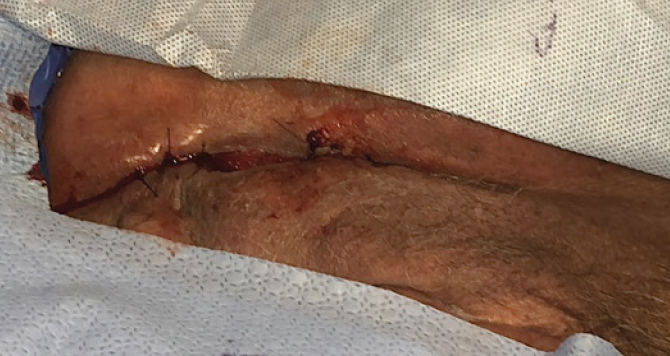
A large necrotizing infection in the perivulvar/perirectal space was debrided to the coccyx. The post-op wound was 15L x 8W x 9D cm. Wound V.A.C. was applied. The wound was re-debrided and irrigated, and negative pressure suction device (NPSD) was applied several times over the next three weeks, prior to the first MiroDerm Fenestrated application
Clinically proven to close hard-to-heal DFUs that had previously failed other advanced therapies1
- Single arm, multi center (9), prospective study
- All patients were non-responsive to at least two applications of one or more advanced biologic wound care product and wounds had not healed within the previous three months
- 53 patients enrolled and 38 completed per protocol over 12 weeks
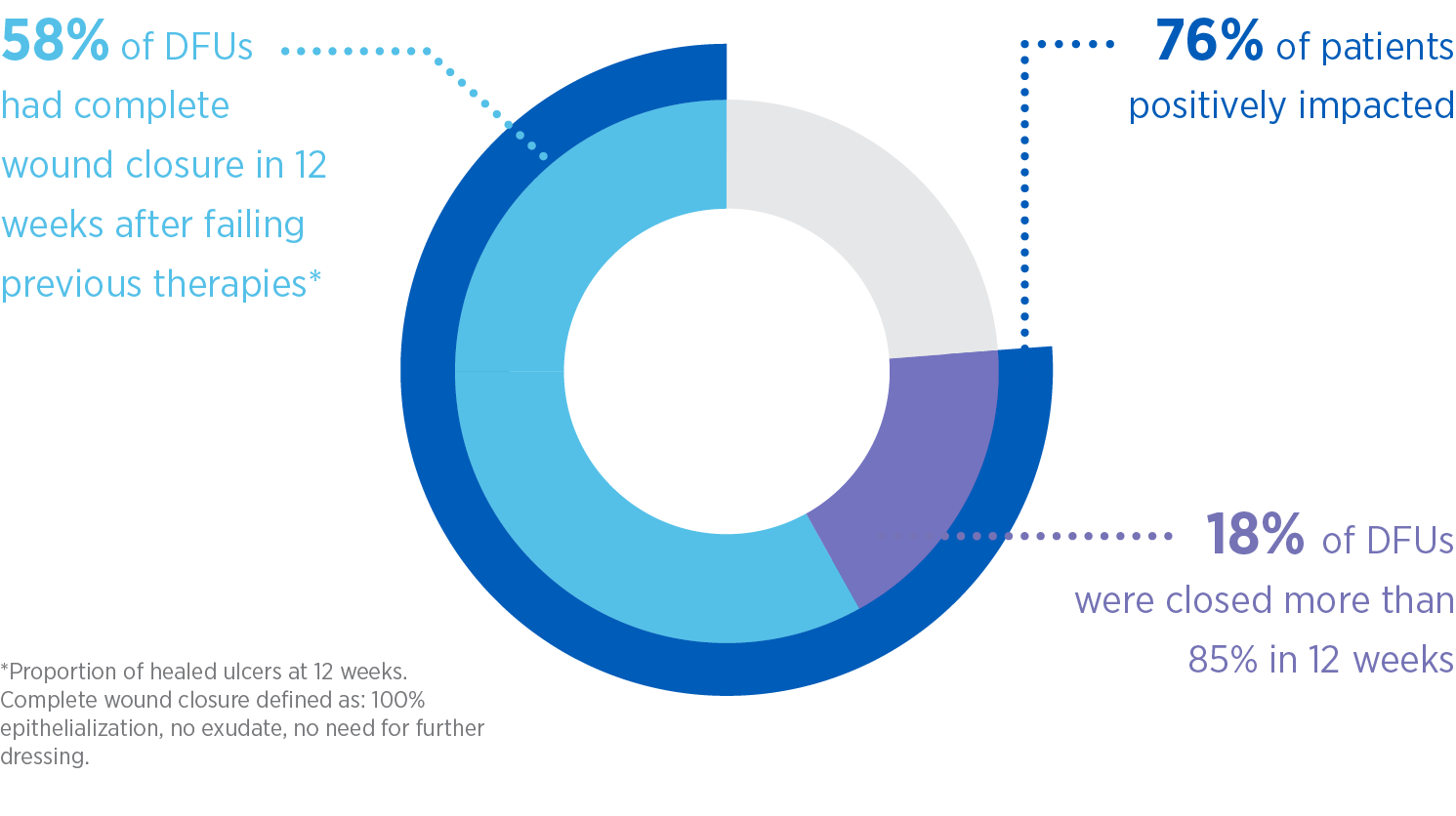
In this study, MiroDerm closed DFUs that had previously failed other advanced therapies. These favorable results are consistent with a similar pilot study.2
Clinical Data
| STUDY | AUTHOR | PUBLICATION | FULL ARTICLE |
| Treatment of Hard-to-heal Diabetic Foot Ulcers With a Hepatic-derived Wound Matrix | Robert Fridman, Payam Rafat, Carl C Van Gils, Deena Horn, Dean Vayser, C Jake Lambert, Jr | Wounds Epub 2020 June 21 |  Read Here |
| A Pilot Study to Evaluate the Effects of Perfusion-decellularized Porcine Hepatic-derived Wound Matrix on Difficult-to-heal Diabetic Foot Ulcers | Robert Fridman and Jonathan Engelhardt | WOUNDS, October 2017 |  Read Here |
Ordering Information
| ITEM ID | SIZE | TOTAL cm2 |
| MiroDerm Fenestrated | ||
| BLM-200-02-0815 | 8 x 15 cm | 120 |
| BLM-200-02-0710 | 7 x 10 cm | 70 |
| BLM-200-02-0808 | 8 x 8 cm | 64 |
| BLM-200-02-0505 | 5 x 5 cm | 25 |
| BLM-200-02-0307 | 3 x 7 cm | 21 |
| BLM-200-02-0404 | 4 x 4 cm | 16 |
| BLM-200-02-0303 | 3 x 3 cm | 9 |
| BLM-200-02-0203 | 2 x 3 cm | 6 |
| BLM-200-02-0202 | 2 x 2 cm | 4 |
| MiroDerm Fenestrated Plus | ||
| BLM-200-03-0815 | 8 x 15 cm | 120 |
| BLM-200-03-0808 | 8 x 8 cm | 64 |
| BLM-200-03-0505 | 5 x 5 cm | 25 |
| BLM-200-03-0303 | 3 x 3 cm | 9 |
MiroDerm Fenestrated and MiroDerm Fenestrated Plus are processed and stored in a phosphate buffered aqueous solution, packaged in an inner sterile pouch and outer non-sterile pouch, and sterilized with electron beam irradiation. The product comes in a variety of sizes and is intended for use only by trained medical professionals who are familiar with wound procedures and techniques involving the use of a wound matrix. Federal (U.S.A.) law restricts this product to sale by or on the order of a physician.
MiroDerm Biologic Wound Matrix (Fenestrated and Fenestrated Plus) is indicated for the management of wounds, including: partial and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma-wounds (abrasion lacerations, second-degree burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric wound dehiscence).
See IFU for full prescribing information, including indications, contraindications, precautions, potential complications, and recommended application instructions.
References:
- Fridman, R; et al. Treatment of Hard-to-heal Diabetic Foot Ulcers With a Hepatic-derived Wound Matrix. Wounds. 2020.
- Fridman, R; Engelhardt, J. A pilot study to evaluate the effects of perfusion-decellularized porcine hepatic-derived wound matrix on difficult-to-heal diabetic foot ulcers. Wounds. 2017. Vol. 29, No. 10. pp. 318-32.
Product Inquiries and Orders
Call us at: 763-284-6780
Fax us at: 952-856-5085
Product inquiries at: customerservice@reprisebio.com
SM-00155 Rev. G 08/22


